Publications 2024
99-2024:
L. Merkel, A. Setaro, C. Halbig, S. Shimizu, T. Yoshii, H. Nishihara, T. Hilal, G. Algara-Siller, C. Koch, S. Eigler*
Structural Model of Oxidatively Unzipped Narrow Single-Walled Carbon Nanotubes.
Carbon 2024, https://doi.org/10.1016/j.carbon.2024.119454.

98-2024:
M. Yanbaeva, J. Soyka, J. M. Holthoff, P. Rietsch, E. Engelage, A. Ruff, U. Resch-Genger, R. Weiss*, S. Eigler*, S. M. Huber*
Dimethylene-Cyclopropanide Units as Building Blocks for Fluorescence Dyes.
Chem. Eur. J. 2024, https://doi.org/10.1002/chem.202402476.
97-2024:
F. Grote, S. Eigler*
Influence of Lattice Defects on trans-Oligoene Substructure Formation in Graphene.
Chem. Eur. J. 2024, e202401031.

96-2024:
F. Grote, B. I. Weintrub, M. Kreßler, Q. Cao, C. E. Halbig, P. Kusch, K. Bolotin, S. Eigler*
Evidence for Trans-Oligoene Chain Formation in Graphene Induced by Iodine.
Small 2024, https://doi.org/10.1002/smll.202311987.

95–2024:
C. E. Halbig,* B. Mukherjee, S. Eigler, S. Garaj*
Origin of Oxygen in Graphene Oxide Revealed by 17O and 18O Isotopic Labeling.
Journal of the American Chemical Society 2024, 146, 7431–7438.

94–2024:
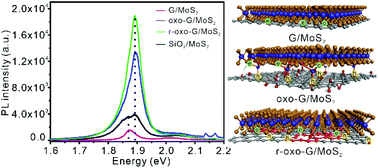
Q. Cao, M. Kreßler, M. Hußmann, Y. Hu, P. Kusch, S. Eigler*
Photoluminescence Modulation of Graphene/MoS2 Heterostructures Separated by Laser-Induced Functionalization.
Chemistry of Materials 2024, 36, 7, 3267–3276; https://doi.org/10.1021/acs.chemmater.3c03166.

93–2024:
S. Trishin, C. Lotze, J. Richter, G. Reecht, N. Krane, P. Rietsch, S. Eigler, K. Franke*
Variations of Vibronic States in Densely Packed Structures of Molecules with Intramolecular Dipoles.
Phys. Status Solidi A 2024, 2300105.

Publications 2023
92–2023:
G. Li, Y. Hu, M. Li,* Y. Tang, Z. Zhang, A. Musiienko, Q. Cao, F. Akhundova, J. Li, K. Prashanthan, F. Yang, P. Janasik, A. N. S. Appiah, S. Trofimov, N. Livakas, S. Zuo, L. Wu, L. Wang, Y. Yang, B. Agyei-Tuffour, R. W. MacQueen, B. Naydenov, T. Unold, E. Unger, E. Aktas,* S. Eigler,* A. Abate*
Managing Excess Lead Iodide with Functionalized Oxo-Graphene Nanosheets for Stable Perovskite Solar Cells.
Angew. Chem. Int. Ed. 2023, 62 (39) e202307395.
Angew. Chem. 2023, 135 (39), e202307395.

91–2023:
S. M. Brülls, V. Cantatore, P. Lam Tam, P. Malmberg, E. Ahlberg, I. Panas, S. Eigler, J. Mårtensson*
Bonding between π-Conjugated Polycations and Monolayer Graphene: Decisive Role of Anions.
J. Phys. Chem. C 2023, 127, 1917–1928.

90–2023:
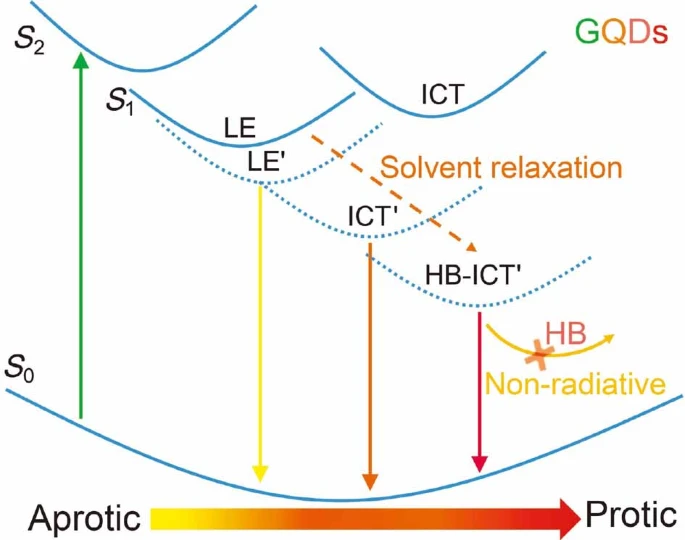
Y. Hu, C. Neumann, L. Scholtz, A. Turchanin, U. Resch-Genger*, S. Eigler*
Polarity, intramolecular charge transfer, and hydrogen bond co-mediated solvent effects on the optical properties of graphene quantum dots.
Publications 2022
89–2022:
S. Trishin, T. Müller, D. Rolf, C. Lotze, P. Rietsch, S. Eigler, B. Meyer, K. Franke
Resolution of intramolecular dipoles and push-back effect of individuals molecules on a metal surface.
J. Phys. Chem. C 2022, 126, 17, 7667–7673

88–2022:
G. Reina, C. Gabellini, M. Maranska, F. Grote, S. M. Chin, L. Jacquemin, F. Berger, P. Posocco, S. Eigler, A. Bianco
The importance of molecular structure and functionalization of oxo-graphene sheets for gene silencing.

87–2022:
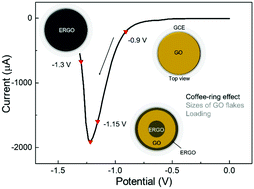
Y. Wang, S. Eigler*
Influence of the coffee-ring effect and size of flakes of graphene oxide films on the electrochemical reduction.
Phys. Chem. Chem. Phys. 2022, 24, 8076-8080.
Publications 2021
86–2021:
Y. Wang, F. Grote, Q. Cao, S. Eigler*
Regiochemically Oxo-functionalized Graphene, Guided by Defect Sites, as Catalyst for Oxygen Reduction to Hydrogen Peroxide
J. Phys. Chem. Lett. 2021, 12, 10009−10014.

85–2021:
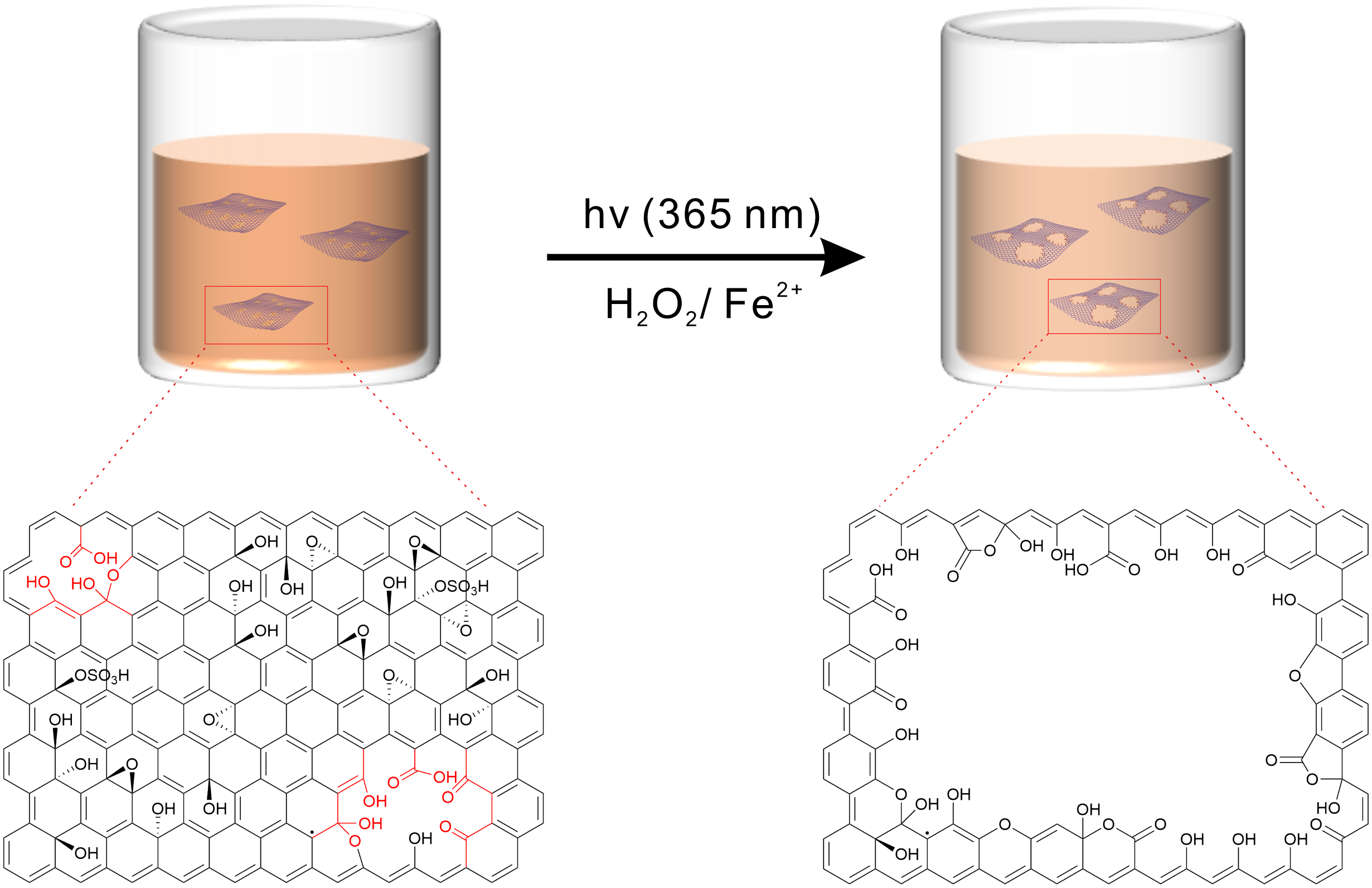
Y. Hu, Q. Cao, C. Neumann, T. Lehnert, F. Börrnert, Y. Wang, U. Kaiser, A. Turchanin, S. Eigler*
Wet-Chemical Synthesis of Solution-Processible Porous Graphene via Defect-Driven Etching
84–2021:
Z. Wang, Q. Cao, K. Sotthewes, Y. Hu, H. S. Shin*, S. Eigler*
Interlayer electron modulation in van der Waals heterostructures assembled by stacking monolayer MoS2 onto monolayer graphene with different electron transfer ability
Nanoscale, 2021, 13, 15464-15470.
83–2021:
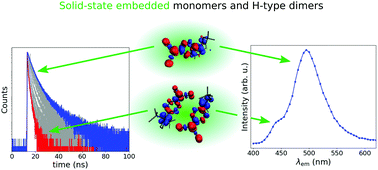
F. Witte, * P. Rietsch, N. Nirmalananthan-Budau, F. Weigert, J. P. Götze, U. Resch-Genger, S. Eigler, B. Paulus
Aggregation-induced emission leading to two distinct emissive species in the solid-state structure of high-dipole organic chromophores
Phys. Chem. Chem. Phys., 2021, 23, 17521.
82–2021:
Y. Wang, C. Neumann, M. Hußmann, Q. Cao, Y. Hu, O. Garrity, P. Kusch, A. Turchanin, S. Eigler *
Synthesis of wet-chemically prepared porous-graphene single layers on Si/SiO2 substrate increasing the photoluminescence of MoS2 in heterostructures
Highlighted in Advanced Materials: Hot Topic: Carbon, Graphite and Graphene
Highlighted in ChemSumChem: Hot Topic: Membranes
81–2021:
S. S. Nugroho, M. N. K. Wihadi, F. Grote, S. Eigler, S. Nakashima*
Potentiality of Graphene Oxide and Polyoxometalate as Radionuclides Adsorbent to Restore the Environment after Fukushima Disaster: A Mini Review
Indonesian J. Chem., 2021, DOI: https://doi.org/10.22146/ijc.60493
80–2021:
H. Liu, G. He, X. Liu, Y. Zhu, S. Eigler, L. Han*
Ion−Induced Formation of Hierarchically Porous Nitrogen−Doped Carbon Materials with Enhanced Oxygen Reduction
ChemCatChem, 2021, 13, 3112-3118.

79–2021:
F. Witte,* P. Rietsch, S. Sinha, A. R. Krappe, J.-O. Joswig, J. Philipp Götze, N. Nirmalananthan-Budau, U. Resch-Genger, S. Eigler, B. Paulus
Fluorescence Quenching in J-aggregates Through the Formation of Unusual Metastable Dimers
J. Phys. Chem. B, 2021, 125, 4438-4446.

78–2021:
Q. Cao, F. Grote, M. Hußmann, S. Eigler*
77–2021:
P. Rietsch, M. Zeyat, O. Hübner, K. Hoffmann, M. Kutter, A. Paskin, J. Uhlig, D. Lentz,* U. Resch-Genger,* S. Eigler*
Substitution Pattern-Controlled Fluorescence Lifetimes of Fluoranthene Dyes
J. Phys. Chem. B, 2021, 125, 1207

Publications 2020
76–2020:
K. Greben,* S. Kovalchuk, A. M. Valencia, J. Kirchhof, S. Heeg, P. Rietsch, S. Reich, C. Cocchi, S. Eigler, K. Bolotin
In situ functionalization of graphene
75–2020:
P. Rietsch, S. Sobottka, K. Hoffmann, A. Popov, P. Hildebrandt, B. Sarkar, U. Resch-Genger,* S. Eigler*
Between Aromatic and Quinoid Structure: A symmetric UV to Vis/NIR Benzothiadiazole Redox-Switch
Chem. Eur. J., 2020, 26, 17361

74–2020:
Y. Nishina,* S. Eigler*
Chemical and Electrochemical Synthesis of Graphene Oxide – A Generalized View
Nanoscale 2020, 12, 12731-12740.
73–2020:
P. Rietsch, S. Sobottka, K. Hoffmann, P. Hildebrandt, B. Sarkar, U. Resch-Genger,* S. Eigler*
Identification of the Irreversible RedOx Behavior of Highly Fluorescent Benzothiadiazoles
ChemPhotoChem, 2020, 4, 668-673.

72–2020:
Z. Wang, Q. Yao, C. Neumann, F. Börrnert, J. Renner, U. Kaiser, A. Turchanin, H. J. W. Zandvliet, S. Eigler*
Identification of Semiconductive Patches in Thermally Processed Monolayer Oxo‐Functionalized Graphene
Angew. Chem. Int. Ed. 2020, 59, 13657-13662.

71–2020:
S. Brülls, V. Cantatore, Z. Wang, P. L. Tam, P. Malmberg, J. Stubbe, B. Sarkar, I. Panas, J. Mårtensson, S. Eigler*
Evidence for Electron Transfer between Graphene and Non‐ covalently Bound π‐Systems
Chem. Eur. J., 2020, 26, 6694-6702.

70–2020:
I. K. Ilic, K. Leus, J. Schmidt, J. Hwang, M. Maranska, S. Eigler, C. Liedel*
Polymerisation in carbone: a novel method for the synthesis of more sustainable electrodes and their application as cathodes for lithium-organic energy storage materials based on vanillin
ACS Sustainable Chem. Eng., 2020, 8, 3055–3064.

69–2020:
Z. Wang, Q. Yao, S. Eigler*
Room‐temperature transport properties of graphene with defects derived from oxo‐graphene
Chemistry – A European Journal, 2020, 26, 6484-6489.

68–2020:
M. Li, W.-W. Zuo, Q. Wang, K.-L. Wang, M.-P. Zhuo, H. Köbler, C. E. Halbig, S. Eigler,* Y.-G. Yang, X.-Y. Gao, Z.-K. Wang,* Y. Li, A. Abate,*
Ultrathin nanosheets of oxo-functionalized graphene inhibit the ion migration in perovskite solar cells,
Advanced Energy Materials 2020, 4, 1902653.

67–2020:
M. Hußmann, B. Weintrub, P. Feicht, G. Germer, K. I. Bolotin, S. Eigler*
Controlled assembly of artificial 2D materials based on the transfer of oxo-functionalized graphene
Nanoscale Advances 2020, 2, 176-181.
Publications 2019
66–2019:
Z. Wang, Q. Yao, Y. Hu, C. Li, M. Hußmann, B. Weintrub, J. N. Kirchhof, K. Bolotin, T. Taniguchi, K. Watanabe, S. Eigler*
Influence of SiO2 or h-BN substrate on the room-temperature electronic transport in chemically derived single layer graphene
RSC Advances 2019, 9, 38011-38016.
65–2019:
S. E. Wawra, G. Onishchukov, M. Maranska, S. Eigler, J. Walter, W. Peukert,*
A multiwavelength emission detector for analytical ultracentrifugation
Nanoscale Advances 2019, 1, 4422-4432.
64–2019:
P. Rietsch, J. Soyka, S. Brülls, J. Er, K. Hoffmann, J. Beerhues, B. Sarkar, U. Resch-Genger, S. Eigler
Fluorescence of a Chiral Pentaphene Derivative Derived from the Hexabenzocoronene Motif,
Chem. Commun. 2019, 55, 10515-10518.
63–2019:
S. Jõemetsa, K. Spustova, K. Kustanovich, A. Ainla, S. Schindler, S. Eigler, T. Lobovkina, S. L. Avila, A. Jesorka, I. Gözen
Molecular Lipid Films on Microengineering Materials
Langmuir 2019, 35, 10286-10298.

62–2019:
P. Feicht, J. Biskupek, T. E. Gorelik, J. Renner, C. E. Halbig, M. Maranska, F. Puchtler, U. Kaiser, S. Eigler
Brodie or Hummers’ – Oxidation conditions determine the structure of graphene oxide
Chemistry – A European Journal 2019, 25, 8955-8959.

Highlighted in Advanced Materials: Hot Topic: Carbon, Graphite and Graphene
61–2019:
P. Rietsch, F. Witte, S. Sobottka, G. Germer, A. Becker, A. Guttler, B. Sarkar, B. Paulus, U. Resch-Genger, S. Eigler
Diaminodicyanoquinone – A Novel Class of Fluorescent Electron Acceptor Dyes with High Dipole Moments
Angewandte Chemie International Edition 2019, 58, 8235-8239;
Diaminodicyanochinone – Fluoreszenzfarbstoffe mit hohem Dipolmoment und Elektronenakzeptor‐Eigenschaften
Angewandte Chemie 2019, 131, 8321.
60–2019:
L. Han, Y. Sun, S. Li, C. Cheng, C. E. Halbig, P. Feicht, J. L. Hübner, P. Strasser, S. Eigler
In-Plane Carbon Lattice-Defect Regulating Electrochemical Oxygen Reduction to Hydrogen Peroxide Production over Nitrogen-Doped Graphene
ACS Catalysis 2019, 9, 1283-1288.

59–2019:
C. E. Halbig, R. Lasch, J. Krull, A. S. Pirzer, Z. Wang, J. N. Kirchhof, K. I. Bolotin, M. R. Heinrich, S. Eigler
Selective Functionalization of Graphene at Defect-Activated Sites by Arylazocarboxylic tert-Butyl Esters
Angewandte Chemie International Edition 2019, 58, 3599-3603
Selektive Funktionalisierung von Graphen an defektaktivierten Bereichen durch Arylazocarbonsäure‐tert‐butylester
Angewandte Chemie 2019, 131, 3637-3641.

58–2019:
K. W. Silverstein, C. E. Halbig, J. S. Mehta, A. Sharma, S. Eigler, J. M. Mativetsky
Voltage-reduced low-defect graphene oxide: a high conductivity, near-zero temperature coefficient of resistance material
Nanoscale 2019, 11, 3112-3116.
Publications 2018
57–2018:
S. Eigler
Mit Schwefelsäure von Graphit zu Graphen
Nachrichten aus der Chemie 2018, 66, 1150-1152.
56–2018:
A. Khannanov, A. Kiiamov, A. Valimukhametova, D. A. Tayurskii, F. Borrnert, U. Kaiser, S. Eigler, F. G. Vagizov, A. M. Dimiev
Gamma-Iron Phase Stabilized at Room Temperature by Thermally Processed Graphene Oxide
Journal of the American Chemical Society 2018, 140, 9051-9055.

55–2018:
C. E. Halbig, O. Martin, F. Hauke, S. Eigler, A. Hirsch
Oxo-Functionalized Graphene: A Versatile Precursor for Alkylated Graphene Sheets by Reductive Functionalization
Chemistry – A European Journal 2018, 24, 13348-13354.

54–2018:
V. Cantatore, S. Pandit, V. R. S. S. Mokkapati, S. Schindler, S. Eigler, I. Mijakovic, I. Panas
Design strategy of a graphene based bio-sensor for glucose

53–2018:
S. Seiler, C. E. Halbig, F. Grote, P. Rietsch, F. Borrnert, U. Kaiser, B. Meyer, S. Eigler
Effect of friction on oxidative graphite intercalation and high-quality graphene formation
Nature Communications 2018, 9, 836.
52–2018:
M. Feierabend, G. Berghauser, M. Selig, S. Brem, T. Shegai, S. Eigler, E. Malic
Molecule signatures in photoluminescence spectra of transition metal dichalcogenides
Physical Review Materials 2018, 2, 014004.
51–2018:
P. Feicht, S. Eigler*
Defects in Graphene Oxide as Structural Motive
ChemNanoMat, 2018, 4, 244-252.

Publications 2017
50–2017:
T. J. Nacken, C. E. Halbig, S. E. Wawra, C. Damm, S. Romeis, J. Walter, M. J. Tehrani, Y. C. Hu, Y. Ishii, S. Eigler, W. Peukert
Structural factors controlling size reduction of graphene oxide in liquid processing

49–2017:
F. Grote, C. Gruber, F. Borrnert, U. Kaiser, S. Eigler, Thermal Disproportionation of Oxo-Functionalized Graphene
Angewandte Chemie-International Edition 2017, 56, 9222-9225
Thermische Disproportionierung von Oxo-funktionalisiertem Graphen
Angewandte Chemie 2017, 129, 9350-9353.

48–2017:
S. Eigler*, A. Hirsch
Controlled Functionalization of Graphene by Oxo-addends
47–2017:
P. Vecera, S. Eigler, M. Kolesnik-Gray, V. Krstic, A. Vierck, J. Maultzsch, R. A. Schafer, F. Hauke, A. Hirsch
Degree of functionalisation dependence of individual Raman intensities in covalent graphene derivatives
Scientific Reports 2017, 7, 45165.
46–2017:
S. Schindler, F. Vollnhals, C. E. Halbig, H. Marbach, H. P. Steinrück, C. Papp, S. Eigler
Focused electron beam based direct-write fabrication of graphene and amorphous carbon from oxo-functionalized graphene on silicon dioxide
PhysChemChemPhys 2017, 19, 2683-2686.
45–2017:
P. Feicht, R. Siegel, H. Thurn, J. W. Neubauer, M. Seuss, T. Szabo, A. V. Talyzin, C. E. Halbig, S. Eigler, D. A. Kunz, A. Fery, G. Papastavrou, J. Senker, J. Breu
Systematic evaluation of different types of graphene oxide in respect to variations in their in-plane modulus

Publications 2016
44–2016:
B. Butz, C. Dolle, C. E. Halbig, E. Spiecker, S. Eigler
Highly Intact and Pure Oxo-Functionalized Graphene: Synthesis and Electron-Beam-Induced Reduction
Angewandte Chemie International Edition 2016, 55, 15771-15774
Nahezu vollständig intaktes und sauberes oxo-funktionalisiertes Graphen – Synthese und elektronenstrahlinduzierte Reduktion
Angewandte Chemie 2016, 128, 16003-16006.

43–2016:
A. Naumov, F. Grote, M. Overgaard, A. Roth, C. E. Halbig, K. Norgaard, D. M. Guldi, S. Eigler
Graphene Oxide: A One- versus Two-Component Material
Journal of the American Chemical Society 2016, 138, 11445-11448.

42–2016:
H. Pieper, C. E. Halbig, L. Kovbasyuk, M. R. Filipovic, S. Eigler, A. Mokhir
Oxo-Functionalized Graphene as a Cell Membrane Carrier of Nucleic Acid Probes Controlled by Aging
Chemistry – A European Journal 2016, 22, 15389-15395.

41–2016:
Book-Review: Graphene: S. Eigler*
An Introduction to the Fundamentals and Industrial Applications
Angewandte Chemie International Edition, 2016, 55, 5122; Angewandte Chemie, 2016, 128, 5206.

40–2016:
S. Eigler
Controlled Chemistry Approach to the Oxo-Functionalization of Graphene
Chemistry – A European Journal 2016, 22, 7012-7027.

39–2016:
C. Hoch, S. Eigler*, S. Wuttke
GDCh – Trendberichte 2015
Nachrichten aus der Chemie, 2016, 62, 246-254.
38–2016:
C. E. Halbig, T. J. Nacken, J. Walter, C. Damm, S. Eigler, W. Peukert
Quantitative investigation of the fragmentation process and defect density evolution of oxo-functionalized graphene due to ultrasonication and milling
37–2016:
H. Chen, Y. Hou, C. E. Halbig, S. Chen, H. Zhang, N. Li, F. Guo, X. Tang, N. Gasparini, I. Levchuk, S. Kahmann, C. O. Ramirez Quiroz, A. Osvet, S. Eigler, C. J. Brabec
Extending the environmental lifetime of unpackaged perovskite solar cells through interfacial design
J. Mater. Chem. A 2016, 4, 11604-11610.
36–2016:
A. Kahnt, R. Flyunt, S. Naumov, W. Knolle, S. Eigler, R. Hermann, B. Abel
Shedding light on the soft and efficient free radical induced reduction of graphene oxide: hidden mechanisms and energetics
RSC Advances 2016, 6, 68835-68845.
35–2016:
S. M. Beladi-Mousavi, S. Sadaf, L. Walder, M. Gallei, C. Ruttiger, S. Eigler, C. E. Halbig
Poly(vinylferrocene)-Reduced Graphene Oxide as a High Power/High Capacity Cathodic Battery Material
Advanced Energy Materials 2016, 6, 1600108.

34–2016:
R. Flyunt, W. Knolle, A. Kahnt, C. E. Halbig, A. Lotnyk, T. Haupl, A. Prager, S. Eigler, B. Abel
High quality reduced graphene oxide flakes by fast kinetically controlled and clean indirect UV-induced radical reduction
33–2016:
S. Grimm, M. Schweiger, S. Eigler, J. Zaumseil
High-Quality Reduced Graphene Oxide by CVD-Assisted Annealing
The Journal of Physical Chemistry C 2016, 120, 3036-3041.

32–2016:
H. Pieper, S. Chercheja, S. Eigler, C. E. Halbig, M. R. Filipovic, A. Mokhir
Endoperoxides Revealed as Origin of the Toxicity of Graphene Oxide
Angewandte Chemie International Edition 2016, 55, 405-407
Toxizität von Graphenoxid: Endoperoxide als Ursache
Angewandte Chemie 2016, 128, 413-416.

Publications 2015
31–2015:
C. E. Halbig, P. Rietsch, S. Eigler
Towards the synthesis of graphene azide from graphene oxide
Molecules 2015, 20, 21050-21057.
30–2015:
Z. Wang, S. Eigler, Y. Ishii, Y. Hu, C. Papp, O. Lytken, H.-P. Steinrück, M. Halik
A facile approach to synthesize an oxo-functionalized graphene/polymer composite for low-voltage operating memory devices
Journal of Materials Chemistry C 2015, 3, 8595-8604.
29–2015:
S. Eigler
Graphite sulphate – a precursor to graphene
Chemical Communications 2015, 51, 3162-3165.
28–2015:
A. Kahnt, R. Flyunt, C. Laube, W. Knolle, S. Eigler, R. Hermann, S. Naumov, B. Abel
How fast is the reaction of hydrated electrons with graphene oxide in aqueous dispersions?
Nanoscale 2015, 7, 19432-19437.
27–2015:
C. D. Wessendorf, R. Eigler, S. Eigler, J. Hanisch, A. Hirsch, E. Ahlswede
Investigation of pentaarylazafullerenes as acceptor systems for bulk-heterojunction organic solar cells
Solar Energy Materials and Solar Cells 2015, 132, 450-454.
26–2015:
J. Walter, T. J. Nacken, C. Damm, T. Thajudeen, S. Eigler, W. Peukert
Determination of the lateral dimension of graphene oxide nanosheets using analytical ultracentrifugation

Publications 2014
25–2014:
S. Eigler, Oxo-Functionalized Graphene
Zeitschrift für Anorganische und Allgemeine Chemie 2014, 640, 2329.
24–2014:
S. Eigler
Mechanistic insights into the reduction of graphene oxide addressing its surfaces
PhysChemChemPhys 2014, 16, 19832-19835.
23–2014:
S. Eigler, A. Hirsch
Chemistry with graphene and graphene oxide-challenges for synthetic chemists
Angewandte Chemie International Edition 2014, 53, 7720-7738
Chemie an Graphen und Graphenoxid – eine Herausforderung für Synthesechemiker
Angewandte Chemie 2014, 126, 7852-7872.

highlighted in: Spotlights, Chem. Phys. Chem., 2014, 15, 1918.
highlighted in: Spotlights, Chem. Eur. J., 2014, 20, 9160.
highlighted in: ChemInform, 2014, 45, 23.
22–2014:
S. Eigler, F. Hof, M. Enzelberger-Heim, S. Grimm, P. Müller, A. Hirsch
Statistical-Raman-Microscopy and Atomic-Force-Microscopy on Heterogeneous Graphene Obtained after Reduction of Graphene Oxide
Journal of Physical Chemistry C 2014, 118, 7698-7704.

21–2014:
S. Eigler, S. Grimm, A. Hirsch
Investigation of the Thermal Stability of the Carbon Framework of Graphene Oxide
Chemistry – A European Journal 2014, 20, 984-989.

20–2014:
R. Flyunt, W. Knolle, A. Kahnt, S. Eigler, A. Lotnyk, T. Häupl, A. Prager, D. Guldi, B. Abel
Efficient route to high-quality graphene materials: Kinetically controlled electron beam induced reduction of graphene oxide in aqueous dispersion
American Journal of Nano Research and Application 2014, 2, 9-18.
19–2014:
J. Kirschner, Z. Wang, S. Eigler, H. P. Steinrück, C. M. Jager, T. Clark, A. Hirsch, M. Halik
Driving forces for the self-assembly of graphene oxide on organic monolayers
Nanoscale 2014, 6, 11344-11350.
18–2014:
Z. Wang, S. Eigler, M. Halik
Scalable self-assembled reduced graphene oxide transistors on flexible substrate
Applied Physics Letters 2014, 104, 243502.
Publications 2013
17–2013:
S. Eigler, Y. Hu, Y. Ishii, A. Hirsch
Controlled functionalization of graphene oxide with sodium azide Nanoscale 2013, 5, 12136-12139.
16–2013:
S. Eigler, S. Grimm, F. Hof, A. Hirsch
Graphene oxide: A stable carbon framework for functionalization
Journal of Materials Chemistry A 2013, 1, 11559-11562.
15–2013:
S. Eigler, S. Grimm, M. Enzelberger-Heim, P. Müller, A. Hirsch
Graphene Oxide: Efficiency of Reducing Agents
Chemical Communications 2013, 49, 7391-7393.
14–2013:
S. Eigler, C. Dotzer, F. Hof, W. Bauer, A. Hirsch
Sulfur Species in Graphene Oxide
Chemistry – A European Journal 2013, 19, 9490-9496.

13–2013:
S. Eigler, M. Enzelberger-Heim, S. Grimm, P. Hofmann, W. Kroener, A. Geworski, C. Dotzer, M. Rockert, J. Xiao, C. Papp, O. Lytken, H. P. Steinrück, P. Müller, A. Hirsch
Wet chemical synthesis of graphene
Advanced Materials 2013, 25, 3583-3587.

12–2013:
Z. Wang, S. Mohammadzadeh, T. Schmaltz, J. Kirschner, A. Khassanov, S. Eigler, U. Mundloch, C. Backes, H. G. Steinrück, A. Magerl, F. Hauke, A. Hirsch, M. Halik
Region-selective self-assembly of functionalized carbon allotropes from solution
ACS Nano 2013, 7, 11427-11434.

11–2013:
F. Hof, S. Bosch, S. Eigler, F. Hauke, A. Hirsch
New Basic Insight into Reductive Functionalization Sequences of Single Walled Carbon Nanotubes (SWCNTs)
Journal of the American Chemical Society 2013, 135, 18385-18395.

10–2013:
N. V. Kozhemyakina, S. Eigler, R. E. Dinnebier, A. Inayat, W. Schwieger, A. Hirsch
Effect of the structure and morphology of natural, synthetic and post-processed graphites on their dispersibility and electronic properties
Fullerenes, Nanotubes and Carbon Nanostructures 2013, 21, 804-823.
Publications 2004-2012
9–2012:
S. Eigler, C. Dotzer, A. Hirsch
Visualization of defect densities in reduced graphene oxide
8–2012:
S. Eigler, C. Dotzer, A. Hirsch, M. Enzelberger, P. Müller
Formation and Decomposition of CO2 Intercalated Graphene Oxide
Chemistry of Materials 2012, 24, 1276-1282.

7–2009:
S. Eigler
A new parameter based on graphene for characterizing transparent, conductive materials
6–2009:
A. Brausam, S. Eigler, N. Jux, R. van Eldik
Mechanistic Investigations of the Reaction of an Iron(III) Octa-Anionic Porphyrin Complex with Hydrogen Peroxide and the Catalyzed Oxidation of Diammonium-2,2 ‘-azinobis(3-ethylbenzothiazoline-6-sulfonate)
Inorganic Chemistry 2009, 48, 7667-7678.

5–2007:
J. E. Jee, S. Eigler, N. Jux, A. Zahl, R. van Eldik
Influence of an extremely negatively charged porphyrin on the reversible binding kinetics of NO to Fe(III) and the subsequent reductive nitrosylation
Inorganic Chemistry 2007, 46, 3336-3352.

4–2007:
J. E. Jee, S. Eigler, F. Hampel, N. Jux, M. Wolak, A. Zahl, G. Stochel, R. van Eldik
Kinetic and mechanistic studies on the reaction of nitric oxide with a water-soluble octa-anionic iron(III) porphyrin complex
Inorganic Chemistry 2005, 44, 7717-7731.

3–2006:
S. Eigler, N. Jux
Highly functionalized water-soluble porphyrins as heme-type models
Proceedings in 1st European Chemistry Congress 2006, 226.
2–2004:
N. Jux, M. Helmreich, S. Eigler, D. Balbinot
Functionalizing porphyrins: electrostatic aggregation and sensors
Journal of Porphyrins Phthalocyanines 2004, 8, 535.
1–2004:
S. Eigler, N. Jux
Synthesis of a water-soluble cytochrome P450NOR model
Journal of Porphyrins Phthalocyanines 2004, 8, 645.
Patents
3–2015 Preparation Method for Graphene Oxide suitable for Graphene Production
S. Eigler*, Hirsch, A., Friedrich-Alexander-Universität Erlangen-Nürnberg, 2015, EP15202056.6.
2–2012 Preparation Method for Graphene suitable for Graphene Production
S. Eigler*, Hirsch, A., Friedrich-Alexander-Universität Erlangen-Nürnberg, 2012, EP12159480.8.
1–2009 High Molecular Organic Semiconductor Material, High Molecular Organic Semiconductor Thin Film and Organic Semiconductor Device
H. Etori, T. Ebine, K. Matsuda, R. B. Frings, S. Eigler, Dainippon Ink & Chemicals, 2009, JP200909994.

99-2024:
L. Merkel, A. Setaro, C. Halbig, S. Shimizu, T. Yoshii, H. Nishihara, T. Hilal, G. Algara-Siller, C. Koch, S. Eigler*
Structural Model of Oxidatively Unzipped Narrow Single-Walled Carbon Nanotubes.
Carbon 2024, https://doi.org/10.1016/j.carbon.2024.119454.