1.3.1.2 Sedimentology laboratory
Both modern and ancient reefs are a perfect laboratory to study sedimentological processes, such as production of particles and rocks, fracturing and erosion, rounding, transport, fixation of particles by binding, storm sedimentation, biosedimentation, biologically mediated carbonate precipitation and much more. We will come back to that in the reef complex and platform sections of this course, but will highlight some aspects of production already in the next section.
1.3.1.3 Carbonate factories: reef and platforms
Huge carbonate platforms in association with reefs have formed during earth history and continuously form today. Often they are up to several 1000 metres thick. Just imagine, if all the carbon dioxide being bound in limestones and dolomites would not be there in the lithosphere but in our atmosphere. Actually, it was reefs which reduced the greenhouse gas to a tolerable extent, so that higher life could develop. We'll discuss this in more detail in the "Reefs through Earth History"-Section.
Growth rates: Upward growth rates of modern stone corals: from millimeters/year to about 30cm/year. Massiv forms average around 3 mm/year, branching ones may frequently grow 5-10cm/year.
Influence of light: Modern corals grow 90% less during night, 50% less during the cloudy season and develop annual growth rings. This is owing to the fact that modern reef building corals contain unicellular algae as so-called photosymbionts (see the coral biology section for details)
Reef growth: maximum upwards reef growth is around 0.5 - 3 cm/year or around 50 metres of upwards reef growth during a minimum of 1000 to 2000 years. Hey, you are right: a reef can only grow upwards if there is place above it. So either the reef started growing in somewhat deeper waters or it is shallow but sea-level is rising or sea-floor is sinking both of which provides additional accomodation space for the reef. Otherwise the reef may grow outwards (it progrades). We'll discuss all this in detail in the Reef and Platform Architecture section.
Despite this intensive carbonate production, the living biomass of corals is only around 1 vol% (which includes even the about 1 million of photosymbiontic algae per square centimeter exposed tissue.
Carbon Dioxide Control and Climate aspects
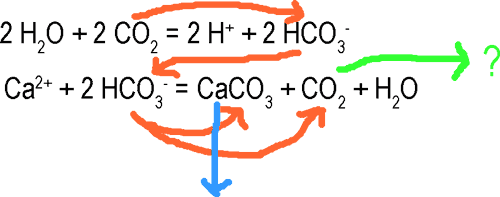
Basic reactions (simplified):
Limestone Precipitation:

orange: pathway of CO2
blue: burial of CO2 in lithosphere
green:free CO2 , in part with undetermined future:
may get dissolved into HCO3-, causing pH-change
may go directly or indirectly (through pH-change) into atmosphere
my be directly used up by photosymbiontsThis formula is very simplified and does not consider feedback with other ions, biotic control and so forth. However, it explains that coral reef and biogenic carbonate sedimentation as a whole is a long-term carbon dioxide sink, transferring CO2 from the connected system atmosphere/hydrosphere into the lithosphere, where it remains mostly immobile (except for melting limestones up in subduction zones, which would cause volcanogenic CO2 expulsion into the atmosphere.
On the other hand, coral reef sedimentation may, on the short term be a source for atmospheric carbon since free CO2 is created which might not be solved in the sea-water but could be directly escape to the atmosphere. Fact is that there is no equilibrium condition between the atmosphere and the hydrosphere, or the upper and lower hydrosphere. This is due to circulation patterns which prevent equilibrium. If there would be no circulation but instead equilibrium, our atmosphere would be a much, much higher CO2-concentration (I believe up to 7 times more, so a specialist has told me, but I do not have a reference for that).
The major problem in discussing whether reef growth may act as short-term atmospheric CO2-source or not can possibly be pinpointed to the following problem:
The free CO2-molecule, created in the process of secretion of a calcareous coral skeleton would immediately stop further carbonate secretion if it would not be used up directly by the symbiontic unicellular algae, the zooxanthellae. The question is whether the zooxanthellae C-reservoir is a continuous one or open to rapid exchange with waters supersaturated in HCO3- which would then expell free CO2 into the atmosphere. If zooxanthellae do represent a stable continuous C-reservoir, global bleaching events (see section ##) would then have a direct impact on climate since their C would be transferred to the atmosphere. In addition, some researches assume that increasing CO2-levels in the atmosphere have a direct negative impact on reef growth, since they may change pH of sea waters (and thus supersaturation) to more acidity, which would slow down biogenic carbonate productivity, a typical positive feedback effect, of if you wish, a vicious cycle.... (see also Kleypas et al. 1999).
Fig. up should show possible pathways of CO2 in marine shallow water systems. Reefs and carbonate platforms transfer CO2 to the lithosphere (as does calcareous nanoplankton in deeper waters, not shown), but may even expell some CO2 into the atmosphere. Since tropical shallow waters are mostly supersaturated in CO2/HCO3- , atmospheric drawdown of CO2 mostly occurs in higher latitudes. Here some dissolved CO2/HCO3- may even be transferred to the lower ocean which is poor in HCO3- . There also might be some circulation between shallow and deeper ocean (more precisely, there is also a medium water layer, which is not shown here for simplification). The important fact is that circulation, and the still open reservoir of the deep ocean prevents equilibrium and hence much higher transfer of CO2 to the atmosphere.
Karstification works the opposite way (simplified):
(SO2 + H2O = H+ + HSO3-) 'acid rain'
CO2 + H2O = H+ + HCO3-
CaCO3 + 2 H+ = CO2 + H+ + Ca2+
more exactly: CaCO3 + H+ + HCO3- = Ca2+ + 2HCO3-
2 HCO3- can remain dissolved in the water (freshwater or sea), so karstification would be a CO2-sink.
Dissolved 2 HCO3- can however dissociate into H2O + CO2, owing to warming of water. CO2 would then escape into the atmosphere, hence in this case karstification would be a CO2-source.
By the way, tropical hydrolytic weathering of silicate rocks is thought to have (and have had) a major influence on the atmospheric CO2-level:
e.g.: CaSiO3 (wollastonite, but could be any other silicate) + 2 H20 + 2CO2 (which yields 2 H+ + 2 HCO3-) = Ca2+ + SiO2*H2O (silica gel) +2HCO3-. This draws down atmospheric CO2 and acts as a sink, as long as the hydrocarbonate remains dissociated. Probably such waters must reach the sea rapidly not to expell their CO2 again to the atmosphere due to warming of river waters. See Historical Geology lecure (no online version yet).
Concluding, the entire carbonate platform - karst system can be both sink and source, depending on the spatial and temporal scales and general conditions. The carbonate-system also buffers water chemistry (weak acids / weak bases), hence preventing rapid pH-shifts which helps stabilizing climate.
Below is a tentative scheme highlighting the carbon cycle. Although numbers (in Gigatons of carbon) are not inequivocal, the importance of biogenic carbonates is clearly indicated. Carbonates, nearly all of which are biogenic, represent by far the largest depot (60 millions gigatons C). Atmosphere contains about 700 to 750 gigatons C, oceans 38.000 gigatons of dissolved C. 3.000 gigatons C are fixed in living organic matter (terrestrial and marine), without counting C fixed in marine animal and plant carbonate skeletons. Fossil fuels are around 5.000 to 10.000 gigatons of C.
Exchange rates are about 120 gigatons C/year on land (photosynthesis versus respiration) and about 90 gigatons C/y above sea (between hydrosphere and atmosphere).
Annual production of C by burning fossil fuel is about 6 gigatons of C per year. It is impressive that this relatively low number has nevertheless risen atmospheric carbon dioxide partial pressure noticeably (from about 250 ppm in the early 50s to now more than 370 ppm).
Fig. and text partially based on Häger et al. 1998, Probst (2000), Schlesinger 1997, Schindler, 1999, and others.
This page is part of www.palaeo.de/edu/reefcourse,
last changes